o Systemic Lupus Erythematosus (SLE)
Definition:
Systemic lupus erythematosus (SLE) is a systemic autoimmune disorder, characterized by a wide spectrum of clinical manifestations that can affect many organs, including the skin, joints, the central nervous system and the kidneys. Rare, inherited, single-gene complement deficiencies are strongly related to SLE, but the disease is inherited during a polygenic manner in most patients. because of the heterogeneity of SLE, Diagnosis remains challenging through a combination of clinical and laboratory criteria.
Panel test
Immunologic tests:
• Complement 3, 4, and CH50 – Low levels of complement in the blood are often linked with lupus.
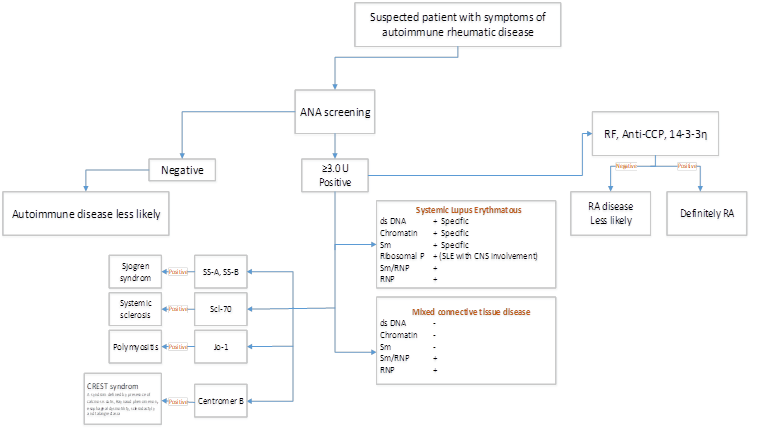
• Antinuclear antibody (ANA) test – it’s an initial screen (not disease-specific); In patients with a positive ANA, more tests are performed for other antibodies that can confirm the diagnosis.
• Double-stranded DNA (dsDNA) – Presence of high antibody titers to native dsDNA is specific and diagnostic for SLE
• Extractable nuclear antigens:
• Smith (Sm) antibodies – highly specific for SLE but occur in only 30-35% of cases
• Ribonucleic protein (RNP) antibodies – not specific for SLE
• Anti-Sjögren’s syndrome antigen A (SSA, or Ro) and anti-Sjögren syndrome antigen B (SSB, or La) antibodies – not specific for SLE
• Histone antibodies – This test is useful for when drug-induced lupus is suspected
• Anti-ribosomal P (anti-P) – related to neurolupus but not useful in diagnosis of neuropsychiatric lupus
• Chromatin antibodies – Primary use – diagnose drug-induced lupus, 50-90% of SLE patients have these antibodies, Related to proteinuria, glomerulonephritis, and disease activity, It’s not specific for SLE, Could be found in Sjögren syndrome and antiphospholipid syndrome (APS)
• Biomarkers of lupus nephritis/disease severity:
• Anti-C1q antibodies – existence of these antibodies can predict risk for severe of disease and it’s related to renal disease.
Consider the following testing to rule out other diseases:
• Antiphospholipid antibodies icluding lupus anticoagulant, anticardiolipin, and anti-beta-2 glycoprotein 1 IgG and IgM assays
• Cryoglobulin
• Neurolupus: CSF is tested for cell count, glucose, oligoclonal bands, culture and Oligoclonal bands, Interleukin 6, 8, 10.
• Collagen type VII antibody IgG – could be positive in bullous SLE
Routine tests:
• Complete blood count. anemia commonly occurs in lupus. A low white blood cell or platelet count may occur in lupus as well.
• Erythrocyte sedimentation rate (ESR) and C-Reactive Protein (CRP). ESR is usually elevated in active SLE, whereas CRP may not be.
• Urinanalysis.
• liver transaminases.
References
1. Fanouriakis A, Kostopoulou M, Alunno A, et al 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus Annals of the Rheumatic Diseases Published Online First: 29 March 2019. doi: 10.1136/annrheumdis-2019-215089
o Sjögren’s syndrome
Definition:
Sjögren’s syndrome (SS), a complex autoimmune rheumatic disease that specifically targets salivary and lachrymal glands, could be a disorder of immune system. It is identified by its two most typical symptoms — dry eyes and a xerostomia. The condition often accompanies other immune system disorders, like rheumatoid arthritis and lupus. In Sjogren’s syndrome, the mucous membranes and moisture-secreting glands of eyes and mouth are usually affected first — cause decreased tears and saliva. Although Sjogren’s syndrome will develop at any age, most of the people are older than 40 at the time of diagnosis. The condition is far more common in women. Treatment focuses on relieving symptoms.
Panel test:
Immunologic tests:
• Antinuclear antibody (ANA): Commonly found in the blood of patients who have Sjogren’s syndrome. This test is useful for initial screen (not disease-specific); In people with a positive ANA, more tests are usually performed to check for other antibodies that can help to confirm the diagnosis.
• Extractable Nuclear Antibody Tests
o anti-SSA (also called anti-Ro) Anti-SSA antibodies are detected in two thirds of Sjögren disease patients.
o anti-SSB (anti-La) is an important component of the ACR/EULAR classification criteria for primary Sjögren syndrome.
• Rheumatoid factor (RF). RF testing is usually used as part of the workup for Sjögren syndrome, as RF is detected in about half of patients
• levels of immunoglobulin. Patients can have hypergammaglobulinemaia
• Novel Autoantibodies:
o anti-salivary gland protein 1 SP-1
o anti-carbonic anhydrase 6 CA-6
o anti-parotid secretory protein PSP- Antibodies (IgG, IgM & IgA of SP-1, CA6 and PSP are helpful markers for diagnosis of patients with SS at early stages of the disease or those that lack antibodies to either Ro or La.
• 2-MG
• Cryoglobulins
References:
1. Mariette X, Criswell LA. Primary Sjögren’s Syndrome. N Engl J Med. 2018; 378(10): 931-939. PubMed
2. Beckman KA, Luchs J, Milner MS. Making the diagnosis of Sjögren’s syndrome in patients with dry eye. Clin Ophthalmol. 2016; 10: 43-53. PubMed
o Scleroderma
Definition:
Systemic sclerosis, also termed scleroderma, is a multisystem connective tissue disease involving autoimmunity, inflammation, fibrosis and vasculopathy. The clinical presentation of systemic sclerosis is variable in patients and characterized by skin thickening, Raynaud’s phenomenon, vasculopathy-induced tissue death and fibrosis of internal organs.
Panel test:
Autoantibodies:
• ANA: It’s especially essential for screening of patients that may have some form of scleroderma.
• Scl-70: anti-topoisomerase 1, Autoantibodies against topoisomerase I (anti-Scl 70 antibodies) are the serologic marker of diffuse cutaneous systemic sclerosis
• Anti-RNA Polymerase III Ab: RNA polymerase III antibodies target RNAP III epitopes 11 and 155. Antibodies to three major components of U1-snRNP (snRNP RNP A, U1-snRNP RNP C, U1-snRNP RNP-70kd): U1- are related to scleroderma and inflammatory myopathy overlap syndromes.
• CENP-A, B and C (anti-centromere antibodies): These antibodies are positive in most of CREST patients (The CREST syndrome: calcinosis, Raynaud’s, oesophageal dysmotility, sclerodactyly, and telangiectasia).
• Anti-U3 RNP (Fibrillarin), Anti-fibrillarin (anti-U3RNP) antibodies are related to diffuse cutaneous SSc, numerous visceral involvement, and particularly renal and cardiac involvement. In some patients, anti-fibrillarin antibodies are associated to severe pulmonary disease, pulmonary hypertension, severe small bowel involvement, and a poor prognosis.
• Th/To: Anti-Th/To antibodies predominantly attach to 2 proteins of the mitochondrial RNA processing (MRP) and also the ribonuclease P complexes, are present in 1-13% of SSc patients, and are rarely detected in other diseases. Anti-Th/To antibodies are primarily associated with localized cutaneous scleroderma, and associated with pericarditis, interstitial lung disease and a high frequency of “intrinsic pulmonary hypertension, and a poorer prognosis.
• PM/Scl-100 and PM/Scl-75: Autoantibodies to PM/Scl, the human exosome complex, are detected in polymyositis/scleroderma overlap syndromes. The majority of anti-PM/Scl reactivity is directed to one of two proteins: PM/Scl100 and/or PM-Scl75.
Other tests
• Immunoglobulins – hypergammaglobulinemia
• Rheumatoid factor, anti-citrullinated antibodies – often positive
References:
1. Knobler R, Moinzadeh P, Hunzelmann N, Kreuter A, Cozzio A, Mouthon L, Cutolo M, Rongioletti F, Denton CP, Rudnicka L, Frasin LA, Smith V, Gabrielli A, Aberer E, Bagot M, Bali G, Bouaziz J, Olesen B, Foeldvari I, Frances C, Jalili A, Just U, Kähäri V, Kárpáti S, Kofoed K, Krasowska D, Olszewska M, Orteu C, Panelius J, Parodi A, Petit A, Quaglino P, Ranki A, Schmidt JM, Seneschal J, Skrok A, Sticherling M, Sunderkötter C, Taieb A, Tanew A, Wolf P, Worm M, Wutte NJ, Krieg T. European Dermatology Forum S1-guideline on the diagnosis and treatment of sclerosing diseases of the skin, Part 1: localized scleroderma, systemic sclerosis and overlap syndromes. J Eur Acad Dermatol Venereol. 2017; 31(9): 1401-1424. PubMed
2. Tartar DM, Chung L, Fiorentino DF. Clinical significance of autoantibodies in dermatomyositis and systemic sclerosis. Clin Dermatol. 2018; 36(4): 508-524. PubMed
o Idiopathic inflammatory myopathies
Definition
The idiopathic inflammatory myopathies are a diverse group of connective tissue diseases of unknown etiology characterized by chronic inflammation of skeletal muscle, or myositis. The most common types of those disorders are consisted of dermatomyositis (DM), polymyositis (PM), necrotizing myopathy (NM) or immune mediated necrotizing myopathy, and sporadic inclusion body myositis (sIBM). Patients ordinarily present with sub-acute to chronic onset of proximal weakness revealed by difficulty with getting up from a chair, climbing stairs, pick up objects, and brushing hair.
Panel test:
• Creatine kinase (CK). elevated in most idiopathic inflammatory myopathies (IIM)
• Aldolase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LD), serum myoglobin- Not usually recommended, Variably elevated, Highly nonspecific
• Thyroid-stimulating hormone – rule out thyroid disease as etiology for muscle weekness
• Antinuclear antibodies Antibody- This test help to rule out connective tissue disease (CTD) or overlap diseases. Staining pattern may be useful in determining the type of confirmatory test(s) to perform. This test could be positive in 50-80% of patients with inflammatory myopathies
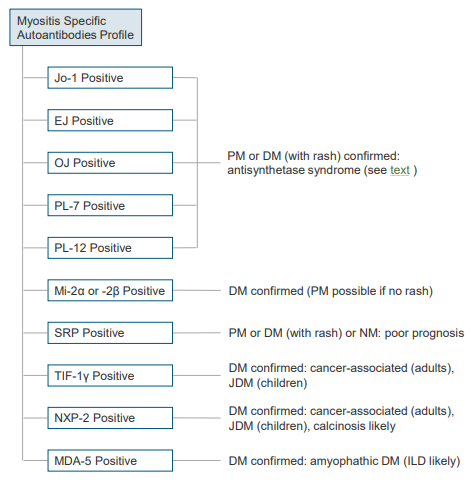
• Antisynthetase antibodies
o anti-Jo-1. Moderate to severe disease, Arthritis common, Low rate of mechanic’s hand
o anti-PL-7. Higher incidence of ILD (interstitial lung disease), Severe arthritis, Raynaud phenomenon, Infrequent myositis
o anti-PL-12. Raynaud phenomenon, more common to have myositis-associated antibodies present concurrently
o anti-EJ. Mechanic’s hands, DM.
o anti-OJ. Arthritis, High incidence of ILD (interstitial lung disease), myositis
• Other myositis-specific antibodies
o Anti SRP (signal recognition particle). Acute, severe necrotizing myopathy
o Severe cardiac involvement
o Anti-Mi-2 (nuclear helicase protein). Classic DM
o Anti P155/140. Aggressive skin lesions in DM
o Anti NXP-2 (nuclear matrix protein-2), DM, Increased malignancy risk, ILD
o Anti TIF1-gamma (TIF1-y), Aggressive skin lesions in DM, Cancer in adults >50 years
o Anti MDA5 (CADM-140), Rapidly progressive ILD, Poor prognosis, CADM
o Anti SAE1 (SUMO activating enzyme), Prognosis is favorable, Initial amyopathic DM, DM, Severe skin disease, dysphagia, and systemic features
o Anti HMGCR. Necrotizing autoimmune myopathy, could be associated with statin therapy
o Anti Mup44. sIBM, also seen in different autoimmune systemic diseases
• Myositis- Associated antibodies- Target autoantigen and overlap syndromes-
o anti-PM/Scl-100. Polymyositis and scleroderma, overlap myositis, MCTD
o anti-SSA(RO). ILD in IM patients
o anti-U1 RNP. MCTD, overlap myositis
o Ku. PM-SSC Overlap
Algorithm
References:
1. Choosing Wisely. An initiative of the ABIM Foundation. [Accessed: Apr 2019]
2. Dalakas MC. Inflammatory muscle diseases. N Engl J Med 2015; 372:1734.
3. O’Connell MJ, Powell T, Brennan D, et al. Whole-body MR imaging in the diagnosis of polymyositis. AJR Am J Roentgenol 2002; 179:967.
4. Lundberg IE. Idiopathic inflammatory myopathies: why do the muscles become weak? Curr Opin Rheumatol 2001; 13:457.
5. Amato AA, Barohn RJ. Evaluation and treatment of inflammatory myopathies. J Neurol Neurosurg Psychiatry 2009; 80:1060.
6. Distad BJ, Amato AA, Weiss MD. Inflammatory myopathies. Curr Treat Options Neurol 2011; 13:119.
o Rhomatoid Arthritis
Definition:
Rheumatoid arthritis could be a chronic inflammatory disorder that may affect over just your joints. In some people, the condition can damage a vast variety of body systems, including the skin, eyes, lungs, heart and blood vessels. An autoimmune disorder, rheumatoid arthritis occurs when your immune system falsely attacks your own body’s tissues.
Despite of the wear-and-tear damage of osteoarthritis, rheumatoid arthritis affects the layers of your joints, causing a painful swelling that can eventually lead to bone erosion and joint deformity.
The inflammation related to rheumatoid arthritis is what can damage other parts of the body as well. While new kinds of medications have improved treatment options dramatically, severe rheumatoid arthritis can still cause physical impairment.
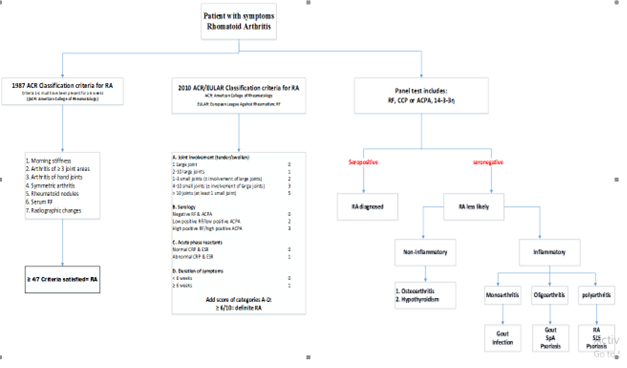
Panel test:
• Antinuclear antibody (ANA) – Commonly found in the blood of people who have lupus, ANAs (abnormal antibodies directed against the cells’ nuclei) can also suggest the presence of polymyositis, scleroderma, Sjogren’s syndrome, mixed connective tissue disease or rheumatoid arthritis. Tests to detect specific subsets of these antibodies can be used to confirm the diagnosis of a particular disease or form of arthritis.
• Rheumatoid factor (RF) –RF is an antibody against the gamma globulin, and it is commonly positive in people with rheumatoid arthritis.
• Anti-cyclic citrullinated peptide (anti-CCP) — Also named anti-citrullinated protein antibodies (ACPA), this test (like the test for rheumatoid factor) looks for the presence of a specific autoantibody that’s present in approximately 60-80 percent of individuals with RA. While most patients with anti-CCP antibodies are positive for RF, the RF antibody can detect in patients with many other situation, including an infection. Anti-CCP is more specific for RA and is becoming the favored test.
• 14.3.3 eta Protein- The 14-3-3η protein be revealed to contribute to the pathologic process of joint erosion and, as such, is an appearing biomarker of joint damage in rheumatoid arthritis (RA) and psoriatic arthritis. Concentrations are significantly higher in people with active joint disease than in those with inactive RA or psoriasis without arthritis. Measurement of 14-3-3η complements RF and CCP antibody tests may improve diagnostic sensitivity.
• RF isotypes
Compounding of RF IgA and CCP may predict radiological damage in RA
When screening for RA, IgM-RF and CCP assays are preferred to other RF isotypes
• Uric acid – By checking the amount of uric acid in the blood, this test can help doctors diagnose gout, a condition that happens when excess uric acid crystallizes and forms deposits in the joints and other tissues, causing inflammation and serous pain.
• HLA tissue typing – Detection of certain genetic markers, can often confirm a diagnosis of ankylosing spondylitis (a disease involving inflammation of the spine and sacroiliac joint) or reactive arthritis (a disease involving inflammation of the urethra, eyes and joints). The genetic marker HLA-B27 is mostly always present in people with either of these diseases.
• CBC and Erythrocyte sedimentation rate There are many conditions that can cause an elevated ESR, including an infection or anemia.
• C-reactive protein that increases in the presence of inflammation.
• Joint fluid tests – like drawing blood, the doctor inserts a needle into a joint space and pull out the fluid. An examination of the fluid may reveal uric acid crystals, confirming a diagnosis of gout; Bacteria cultured from joint fluid can indicate that the joint inflammation is caused by an infection.
Algorithm
References
1. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Ménard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovský J, Wolfe F, Hawker G. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010; 62(9): 2569-81. PubMed
2. Choosing Wisely. An initiative of the ABIM Foundation. [Accessed: Apr 2019]
3. van der Heijde D, van Mil AH, Aletaha D, Bingham CO, Burmester GR, Dougados M, Emery P, Felson D, Knevel R, Kvien TK, Landewé RB, Lukas C, McInnes I, Silman AJ, Smolen JS, Stanislawska-Biernat E, Zink A, Combe B. EULAR definition of erosive disease in light of the 2010 ACR/EULAR rheumatoid arthritis classification criteria Ann Rheum Dis. 2013; 72(4): 479-81. PubMed
o Mixed connective tissue disorders
Definition
Mixed connective tissue disease (MTCD) is an unusual systemic inflammatory rheumatic disease. MCTD could be a specific subset of the wider category of rheumatic “overlap syndromes”, a term used to describe when a patient has features of over than one classic inflammatory rheumatic disease. These classic rheumatic diseases consist of systemic lupus erythematosus, polymyositis, scleroderma, and rheumatoid arthritis.
• Routine tests:
o CBC
o ESR
o CRP
o Routine blood chemistry
o Urinalysis
• Immunology tests
• Autoantibodies:
o ANA
o RNP- Positive in 95-100% MCTD patients
o dsDNA, Sm, Ribosomal-P- present in MCTD patients
• Other tests:
o Immunoglobulins- hypergammaglobulinemia
o Rheumatoid factor- often positive
o Anti-CCP- often positive
Algorithm
References:
1. Sharp GC, Irvin WS, Tan EM, et al. Mixed connective tissue disease–an apparently distinct rheumatic disease syndrome associated with a specific antibody to an Extractable Nuclear Antigen (ENA). Am J Med 1972; 52:148–59.
2. Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. Can Med Assoc J 2010;182: E839–42.
3. Benito-Garcia E, Schur PH, Lahita R, et al. Guidelines for immunologic laboratory testing in the rheumatic diseases: anti-Sm and anti-RNP antibody tests. Arthritis Rheum 2004; 51:1030–44.